Perfluoralkylsulfones
Synthesis and Application of Perfluoroalkylsulfones
Since more than 15 years we are interested in the preparative application of
perfluoroalkanesulfonyl groups in organic chemistry. Double bonds substituted with strong
electron - attracting perfluoroalkanesulfonyl group are well activated and are therefor suitable
for Diels - Alder reaction.The perfluoroalkyl sulfone products formed can be used further
preparatively or the perfluoralkylgroup can be replaced wifth hydrogen.
There are several reviews and a textbook [1]
on the
chemistry of sulfones, however, they all deal mainly with phenyl or arylsulfones. As one of the
first research groups, we have successfully used the strong perfluoroalkanesulfonyl group in
different reactions. in general, the synthetic potential of trifluoromethanesulfones (triflones)
and nonafluoromethanesulfones (nonaflone) were systematically studied.
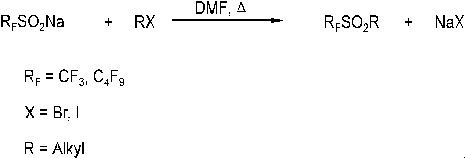
Perfluoroalkanesulfones were prepared form the corresponding perfluoroalkanesulfonic acid
sodium salt and appropriate alkyl halides (Scheme 1). Perfluoroalkanesulfonyl substituted
alkenes and alkynes were prepared according to Scheme 2
[2-5]
.
Scheme 1:
Scheme 2:
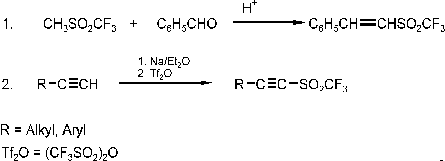
The influence of perfluoroalkansulfonyl groups to activitate the C-C- single und double bonds
has been systematically investigated by us [6-8]
These studies show ethenyl perfluoroalkansulfones to be strong dieneophiles capable of
undergoing Diels- Alder reaction already at room temperature. Alkynyl sulfones are found to
undergo easy nucleophilie substitution (Scheme 3).
Scheme 3:

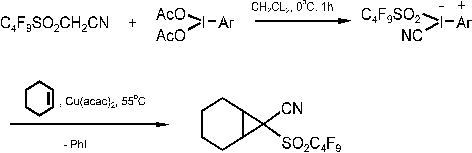
Recently, we have successfully generated perfluoroalkanesulfonyl substituted carbenes
according to Scheme 4 [9]
.
Scheme 4:
During the studies on the very reactive phenyliodoniumethoxynonaflylmethanide, we
discovered two new reactions, e.g. the formation of
 - lactons
[10]
(Scheme 5).
- lactons
[10]
(Scheme 5).
Scheme 5:
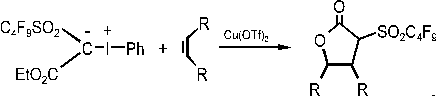
 Top of the Page
Top of the Page

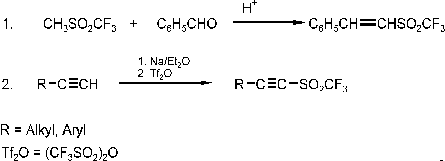


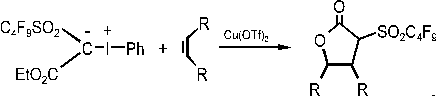
 Top of the Page
Top of the Page